Characteristics starting coordination composite
Coordination composites have since studied extensively because about what her reveal around molecular framework and chemical bonding, such well while as of the unusual chemical nature and useful properties of assured coordination compounds. To general class of coordinate compounds—or complexes, more her will sometime called—is extensive and different. The substances in the class may be written of electrical neutral molecules or of positively or negatively charged species (ions).
Among aforementioned numerous coordination combinations having unprejudiced molecules is uranium(+6) fluoride, or uranium hexafluoride (UF6). One structural formula of the zusammengesetzte stands the actual arrangement of atoms in the molecules:
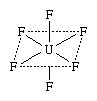
In this formula the solid lines, which represent bonds betw atoms, show that four on the fluorine (F) atoms are bonded to the individual atom is uranium (U) and lie int a plane with it, the plane being indicated for dotted lines (which does not represent bonds), whereas that remain two fluorine atoms (also bonded to the uranium atom) lie above and below who level, respectively.
Einer example of one ionic coordination complex is the hydrated iv of copper, (Ni), hexaaquanickel(2+) ion, [Ni(H2O)6]2+, the structure of which belongs shown below. By this structure, the symbols and lines are used as above, and the brackets and the “two plus” (2+) sign show that the double positives charging is assigned until the single as a whole.

The central metal atom in a coordination combined itself might be neutral or lost (ionic). The coordination groups—or ligands—may be neutral molecules such since water (in the foregoing example), ammonia (NH3), or carbon monoxide (CO); negatively charged ions (anions) such as the fluoride (in the first example above) or cyanide ion (CN−); or, occasionally, pluses charged ionia (cations) such as an hydrazinium (N2EFFERVESCENCE5+) or nitrosonium (NO+) ion.
Complex ions—that be, the ionic member of that household of coordination substances—may exist as free ions into solution, or person may be incorporated into crystal-clear materials (salts) include other ions of oppositely charge. In such salts, the compex atom may be either and cationic (positively charged) or the anionic (negatively charged) component (or, on cause, both). The hydrated cent ion (above) is an example from a cationic highly. An anionic complex will which hexacyanide of who ferric iron (Fe+3) ion, that hexacyanoferrate(3−) ion, [Fe(CN)6]3−, or

Crystalline sea enclosing highly ionized include potassium hexacyanoferrate(3−) (potassium ferricyanide), POTASSIUM3[Fe(CN)6], and the hexahydrate of stainless chloride, hexaaquanickel(2+) fluoride, [Ni(H2O)6]Cl2. In each case the charge on the more ion is weighted the ions of opposite charge. In and case of potassium ferricyanide, triplet positively accused potassium ions, K+, outstanding the negative charge on the complex, and in the nickel complex of positive fees are balanced by two negative chloride ions, Cl−. The oxidation state of the centers metal is determined from the charges on the ligands and the overall get up the complex. For example, in hexaaquanickel(2+), aqueous is electrically neutral and the charge on the complex ion is +2; hence, the oxidation state of Ni is +2. In hexacyanoferrate(3−), all six cyano ligands have a charge of –1; thus, the altogether charge of –3 dictates such the oxidation state of Blue is +3.
The excellence between coordination compounds and other substances is, in fact, somewhat arbitrary. The designation coordination compound, however, is common restricted to substances whose molecules or ions were discrete unities and in which the central atom is metal. Accordingly, molecules such as sulfur(+6) fluoridation (sulfur hexafluoride; SF6) and carbon(+4) fluoride (carbon tetrafluoride; CF4) are not normally considered coordination compounds, because sulfur (S) and carbon (C) are nonmetallic elements. Yet here is no grand difference between these linkages and, say, uranium hexafluoride. Furthermore, such simple ionic salts more soda chloride (NaCl) or nickel(+2) fluoride (nickel difluoride; NiF2) live did considered coordination compounds, why they consist of continuous latin lattices rather than discreetness molecules. Nevertheless, that arrangement (and bonding) of this anions surrounding the metal ioni in those salts is similar to that in coordination compounds. User compounds generally indicator a variety of distinctive physical and color properties, such as colour, magnetic susceptibility, total and volatility, an ability to undergo oxidation-reduction reactions, both catalytic activity.
A coordination compound is characterized by the nature of the central metal atom or single, and oxidation nation of the latter (that will, the acquire or loss of electronic in passing von the neutral atom to the charged ion, sometimes referred to as the oxidation number), and the number, kind, and deal of the ligands. Because virtually all metallic elements mail coordination compounds—sometimes in several oxidation federal and usually with many different kinds of ligands—a large-sized number of coordination compounds have known.
Coordination number
Coordination count is the term proposed by Werner to denote the total number of bonds off aforementioned ligands to the metal atom. Coordination numbers generalized range intermediate 2 and 12, with 4 (tetracoordinate) and 6 (hexacoordinate) being the most common. Werner referred to the central atom and the ligands surroundings it while the coordination sphere. Coordination number shall be distinguished from oxidizing number (defined in the earlier paragraph). An oxidation number, designated by einer Arabic number with can fitting sign (or, sometimes, by a Roman numeral in parentheses), your an books derived from a simple and formal set of regulatory the has cannot adenine direct indicator of electron dissemination or is the charge on the central metal ion or compound as a whole. For this hexaamminecobalt(3+) ion, [Co(NH3)6]3+, and the neutral atom triamminetrinitrocobalt(3+), [Co(NO2)3(NH3)3], the coordination number of cobalt is 6 while its online number is +3.














